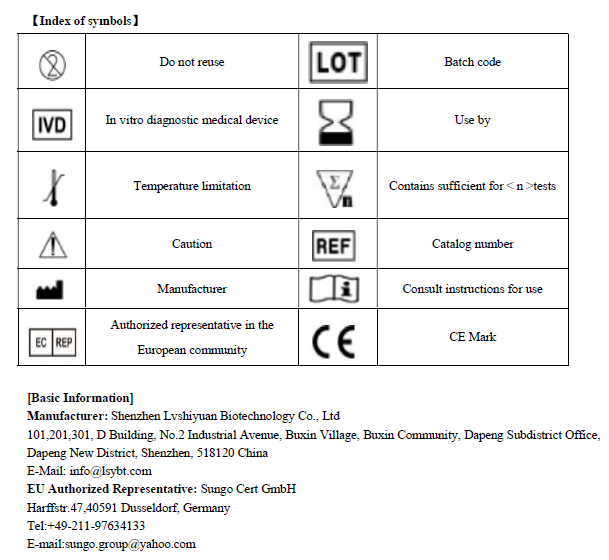
Group A streptococcus (Strep A) Test Kit (Colloidal
Gold Method)
【Product Name】 Group A streptococcus (Strep A) Test Kit (Colloidal
Gold Method)
【Packing Specifications】 25 servings/box
【Intended Use】
Strep A Test Kit is a rapid and convenient
immunochromatographic assay for the qualitative detection of Group A
streptococcus (Strep A) antigen from patient throat swab specimens. It is
intended for professional use as an aid in the diagnosis of Strep A infection.
This assay provides only a preliminary result. Clinical expertise and
professional judgment should be sought to further evaluate the result of the
test.
【Test Principle】
Streptococcus pyogenes is non-motile
gram-positive cocci and known as Group A Streptococcus. The bacteria cause
serious acute upper respiratory tract infections such as pharyngitis,
respiratory infection, impetigo, endocarditis, meningitis, sepsis and
arthritis. Identification procedures in laboratory for Strep A infection
involve isolation of viable organisms, followed by various biochemical,
microbiological (cell culture) or molecular biology techniques that usually
require 24 to 48 hours or even longer. Early diagnosis and treatment of Group A
Streptococcal pharyngitis has been shown to reduce the severity of symptoms and
further complications such as rheumatic fever and glomerulonephritis. Strep A
Test detects either viable or nonviable organisms directly from a throat swab,
providing results within 10 minutes.
The test is an antigen-capture
immunochromatographic assay, which detects the presence of Strep A in throat
swab samples. Monoclonal antibodies specifically against Strep A are 1)
conjugated with colloidal gold and deposited on the conjugate pad and 2)
immobilized on the test line of the nitrocellulose membrane. When adequate
volumes of the test sample is added the antibody conjugate is rehydrated and
the Strep A, if any in the samples, will interact with the colloidal gold
conjugated antibodies. The antigen-antibody-gold complex will migrate towards
the test window until the Test Zone (T) where they will be captured by
immobilized antibodies, forming a visible pink line (Test band), indicating a
positive result. If Strep A are absent in the sample, no pink line will appear
in the Test Zone (T), indicating a negative result.
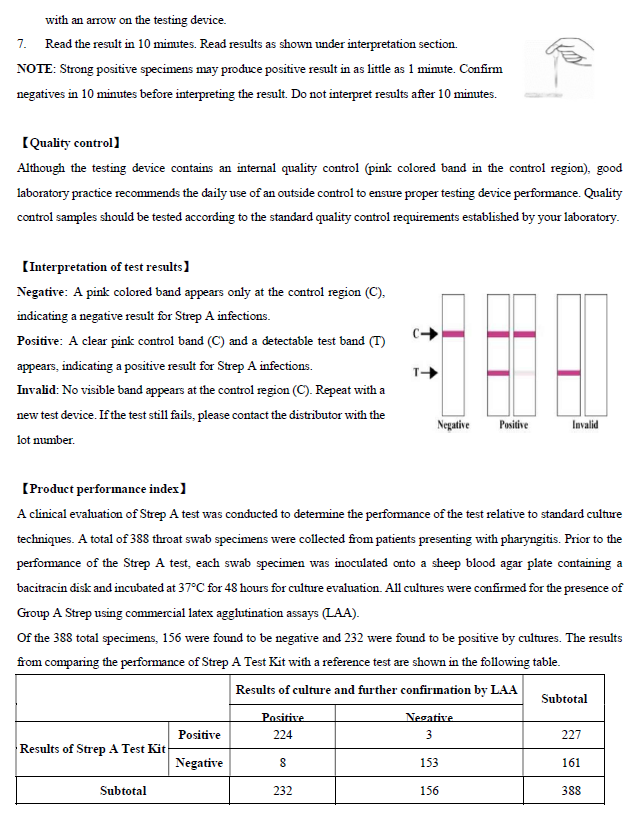
To serve as an internal process control, a
control line should always appear at Control Zone (C) after the test is
completed. Absence of a pink control line in the Control Zone is an indication
of an invalid result. The detection limit of Strep A Test Kit is approximately
105 Group A Streptococci organism.
【Main components】
1.
Pouch
contents: Test cassette, desiccant, sample dropper.
2. Sample extraction buffer A (7 ml per bottle
for 25 tests), buffer B (8 ml per bottle for 25 tests).
3. Test instruction.
【Storage and stability】
1.
Test device in
the sealed pouch should be stored at 2~30°C. Do not freeze the test device.
2. The bottle containing the buffer should be stored at 2~30°C.
3. The test device should be kept away from direct sunlight, moisture
and heat.
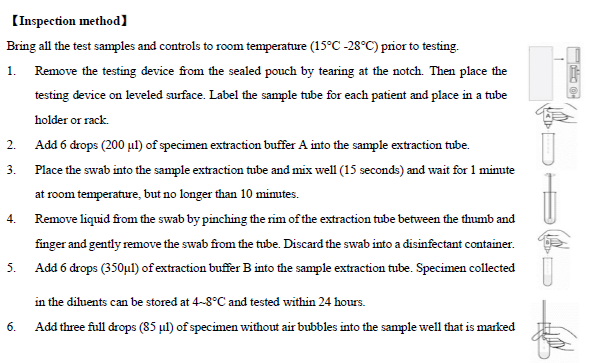
【Sample requirements】
1. Swab the posterior pharynx, tonsils and
other inflamed areas (Note: avoid touching the tongue, cheeks and teeth with
the swab).
2.
A
specimen should be collected by standard throat swab collection methods. It is
preferable to use dacrontipped
sterile swab with plastic shafts. Swabs should be processed as soon as possible
after collection. If swabs are not processed immediately, they should be placed
into a sterile, dry, tightly capped tube or bottle. Swabs which have been
transported in liquid media such as modified Stuart's Transport Medium may be
used in the test provided the liquid medium has a volume of 1 ml or less. Do
not use semisolid transport media containing charcoal. Specimen swabs may be
refrigerated (2-8 °C) for up to 5 days. If a culture is desired,
lightly roll the swab tip onto a Group A selective blood agar plate before
using the swab in the Strep A Rapid Test Device
3.
Add
three full drops (85 µl) of specimen without air bubbles into the sample well that
is marked with an arrow on the testing device.
Diagnostic
Sensitivity = 96.55%
Diagnostic
Specificity = 98.08%
Accuracy
= 97.16%
【Limitations of the inspection method】
1.
Humidity
and temperature can adversely affect results.
2. The instructions for the use of the test
should be followed during testing procedures.
3.
There
is always a possibility that false results will occur due to the presence of
interfering substances in the specimen or factors beyond the control of the
manufacturer, such as technical or procedural errors associated with the
testing.
4.
Although
the test demonstrates superior accuracy in detecting Strep A, a low incidence
of false results can occur. Therefore, other clinically available tests are
required in case of questionable results. As with all diagnostic tests, a
definitive clinical diagnosis should not be based on the results of a single
test but should only be made by the physician after all clinical and laboratory
findings have been evaluated.
【Precautions】
1.
For professional in vitro diagnostic use only. Do not reuse.
2. Do not use if the product seal or its packaging is compromised.
3. Do not use after the expiration date shown on the pouch.
4. Do not mix and interchange different specimens.
5.
Wear
protective clothing such as laboratory coats, disposable gloves and eye
protection while handling potentially infectious materials or performing the
assay.
6. Wash hands thoroughly after finishing the tests.
7. Do not eat, drink or smoke in the area where the specimens or kits
are handled.
8. Clean up spills thoroughly with appropriate disinfectants.
9. Handle all specimens as if they contain infectious agents. Observe
established precautions against microbiological hazards throughout testing
procedures.
10. Dispose of all specimens and used devices in a proper bio-hazard
container. The handling and disposal of the hazardous materials should follow
local, national or regional regulations.
11. Keep out of children's reach.