Helicobacter Pylori Antigen (HP-Ag) Rapid Test Kit (Colloidal Gold)
【Product Name】
Generic Name: Helicobacter Pylori Antigen (HP-Ag) Rapid Test Kit (Colloidal
Gold)
[Packing
Specification] 25 tests/package
[Intended
Use] Helicobacter Pylori Antigen
(HP-Ag) Rapid Test Kit (Colloidal Gold) is used for the rapid qualitative detection of HP-Ag in the stool to
assist in the diagnosis of HP infection in patients with gastric symptoms. Some
studies have shown that HP infection is a cause for chronic gastritis, peptic
ulcerative gastric adenocarcinoma and low-grade gastric lymphoma, so the
clinical detection of HP injection is of great significance.
[Test principle] Double antibody sandwich
immunochromatography is adopted to qualitatively detect HP in the stool. HP
monoclonal antibody is coated on nitrocellulose (NC) membrane, and the other
antibody is bound to the surface of colloidal gold particles. The specimen is
added to the sample hole through the filter membrane coated with colloidal gold
antibody. When HP-Ag is found in the patient’s specimen, it binds to the
gold-labeled HP monoclonal antibody. In
the test line (T) area, the HP-Ab complex begins to contact with the Ab coated
in the T area, forming a sandwich complex of Ab-HP-gold-labeled Ab with a
purplish red band. The Ab labeled by colloidal gold diffuses to the quality
control line (C) area and is captured by the secondary antibody to form a red
band in C area.
[Main Components ]
|
Ingredients
|
Quantity/Specification
|
Main components:
|
|
Test card
|
25 tests/box
|
For each test card, the sample pad is coated
with HP monoclonal Ab labeled by colloidal gold, and the NC membrane part is
coated with another HP monoclonal Ab and test strips for goat-anti-mouse
Ab
|
|
Stool collection tube (containing diluent in
tube)
|
25 tests/package
|
0.02M of PBS buffer solution
|
|
specimen collection container
|
25 tests/package
|
/
|
|
Manual
|
1 copy
|
/
|
Components required for testing but not provided:
1.
timer
2. hygrothermograph
Notes: Components in reagents at different batches cannot be used
interchangeably to avoid errors.
[Storage and Validity] Store at
2℃-30℃ in a cool, dry place away from light, and the validity period is 18
months.
[Applicable Instrument] NA
【Sample Requirements】
◆ Specimen
collection
1. Collected stool specimens must
be collected in a clean, dry, preservative-free, decontamination-free
hydrosolvent container; All specimens should be treated as infectious agents.
2. Collect enough stool specimens (1-2 ml or 1-2 g) to get a
representative sample.
3. Do not keep stool specimens at
room temperature for a long time. If the specimens are free of bacterial
contamination, please use them within 48 hours when stored at 2 to 8 °C; if not
within the time limit, the samples must be stored at -20 °C;
4. Do not use specimens that have
been placed for a long time to avoid non-specific reactions caused by specimen
contamination and bacteria growth;
◆ Specimen
gathering
Unscrew the cover of the stool collection tube, insert the sampling rod
attached to the cover into the stool specimen, rotate it 360 degrees clockwise,
and then put it into the collection tube and shake it well with the specimen
diluent.
【Test Method】
1.
Only
after the restoration to room temperature, the kit and collected test sample of
low-temperature storage can be used.
2.
Tear
off the outer package, take out the test card, and lay it flat on the operating
table
3.
Insert
the sampling rob into the stool specimen, rotate it 360 degrees clockwise, and
then put it into the collection tube, shake it well with the specimen
diluent and then tighten the lid.
4.
Break
off the tip on the cover of the stool collection tube that is with specimens,
put the stool collection tube upside down vertically and drop two drops (about
80 ul) of the specimens into the specimen hole on the test card to start
timing.
5.
Read the final results in 15-20 minutes. But not
20 minutes later.
Attention: The test should be completed at room
temperature of 15-30°C and humidity below 85%.
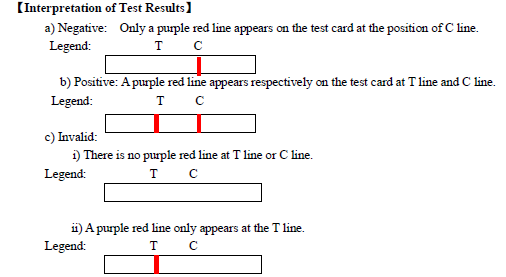
【Limitations of Test Method】
1.
The kit is only for in vitro diagnosis, and only to qualitatively detect HP in stool
specimens, but cannot determine HP content in the specimen.
2.
If there are too few Ag to be tested in the
specimens, it will cause false negatives.
3.
But too much can cause false positive or invalid
result.
4.
The kit is only for primary screening, and the
results cannot be used as the evidence for diagnosis. To ensure the accuracy of
the test results, further diagnosis can be made by gastroscopy combined with
Warthin-starry silver stain and rapid urease test, etc..
【Product Performance Indicators】
1.
Liquid migration time: Use the same batch of kit
to test the internal quality control products, start timing when the liquid is
dripped into the well, and the time required for the liquid to completely pass
through the observation window should be less than 5 minutes.
2.
Sensitivity: The sensitivity quality control
products provided by the manufacturer shall be used as samples for testing, and
the results shall meet the requirements.
3.
Specificity: Test with the specific quality control
product provided by the manufacturer, and the test results should be
consistently negative;
4.
Intra-batch repeatability: 10 test cards are
randomly selected from the same batch of kits, and the repeated quality control
products provided by the manufacturer are used as samples for parallel testing,
and the test results should be consistently positive.
5.
Inter-batch repeatability: 10 test cards are randomly selected from
three consecutive batches of kits (at least 3 cards per batch), and the
repeated quality control products provided by the manufacturer are used as
samples for parallel testing, and the test results should be consistently
positive.
6.
Stability: The kit is stored under the required
storage conditions for three months beyond the validity period, the specified
tests are carried out, and the results should meet the requirements.
【Precautions】
1.
This kit is used only for in vitro diagnosis,
and can not be reused.
2.
The kits should be treated as the one containing
infectious materials, and the waste after use should be disposed of in
accordance with relevant regulations.
3. Helicobacter Pylori Antigen (HP-Ag) Rapid Test Kit (Colloidal Gold) is for
testing stool specimens.
4.
During the interpretation time, regardless of
the color of the band, as long as two lines can be observed in C area and T
area, it can be judged as positive.
5.
Please ensure that an appropriate amount of
specimens is used for testing, too much or too little will lead to deviation.
6.
Read test results at 15-20 minutes, and it is
invalid after 20 minutes.
【References】
1.
Fan Wenwei, Du Jianfeng, Liu Shengqi. Clinical
application of fecal Helicobacter pylori antigen determination. Chinese Journal of Modern Clinical Medicine,
Vol. 3, No. 23, 2005
2.
Yan Wei, Cao Jianbiao. Progress in the test
technology of gastric Helicobacter pylori. World Chinese Journal of Digestion, May 28, 2009; 17(15)
3.
Hu Fulian, Zhou Dianyuan. The basis and clinic
of Helicobacter pylori infection (third edition). Beijing: China Science and Technology Press, 2009
[Basic
Information]
Manufacturer: Shenzhen Lvshiyuan Biotechnology Co., Ltd
101,201,301, D
Building, No.2 Industrial Avenue, Buxin Village, Buxin Community, Dapeng
Subdistrict Office, Dapeng New District, Shenzhen, 518120 China
E-Mail: info@lsybt.com
EU Authorized Representative: Sungo Cert GmbH
Harffstr.47,40591
Dusseldorf, Germany
Tel:+49-211-97634133
E-mail:sungo.group@yahoo.com